Background: Ibrutinib is the only once-daily Bruton's tyrosine kinase inhibitor approved in the US and EU either as single-agent therapy or in combination with rituximab for treatment of patients with Waldenström's macroglobulinemia (WM) across all lines of therapy. The open-label substudy of the phase 3 iNNOVATE trial (PCYC-1127; NCT02165397) demonstrated that single-agent ibrutinib was highly efficacious (90% overall response rate [ORR] per investigator) with improved responses over time in heavily pretreated, rituximab-refractory patients with WM (Buske Blood 2018). Here, we present results from the final analysis of the iNNOVATE open-label substudy.
Methods: Patients with WM who failed to achieve at least a minor response (MR) or who relapsed <12 months after their last rituximab-containing therapy received once-daily ibrutinib 420 mg. Endpoints included progression-free survival (PFS) and ORR (≥MR) per Independent Review Committee (IRC), overall survival (OS), hemoglobin (Hgb) improvement, and safety; serum immunoglobulin M (IgM) reduction was also assessed.
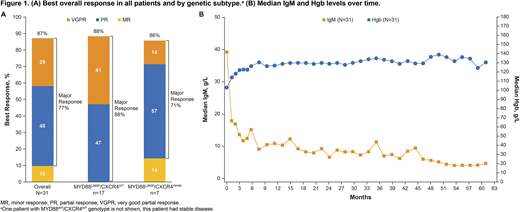
Results: Thirty-one patients with rituximab-refractory disease were enrolled; median age was 67 years (range 47-90), and median number of prior therapies was 4 (range 1-7). Median baseline Hgb was 103 g/L (range 64-146). Median baseline IgM was 39 g/L (range 9-107). Median follow-up was 58 months (range 9-61). Median PFS was 39 months (95% CI 25-NE); the PFS rate at 60 months was 40%. Median PFS was not reached (95% CI 27-NE) in patients with the MYD88L265P/CXCR4WT genotype and was 18 months (95% CI 3-28) in patients with the MYD88L265P/CXCR4WHIM genotype. ORR was 87%, with similar ORR observed across genetic subtypes (MYD88L265P/CXCR4WT, 88% [15/17]; MYD88L265P/CXCR4WHIM, 86% [6/7]); major response rates (≥PR) among these genotypes were 88% (15/17) and 71% (5/7), respectively (Figure 1A). Median OS was not reached in the full cohort of patients, regardless of number of prior therapies (1-2 vs ≥3). Improvements in IgM and Hgb were generally rapid and sustained (Figure 1B). Twenty-two patients (71%) had sustained improvement in Hgb, including 17/21 (81%) with baseline Hgb ≤110 g/L. Median change in IgM from baseline to nadir (month 54) was -37 g/L (range -75 to -5). Median duration of ibrutinib treatment was 41 months. The most common reason for discontinuing ibrutinib while on study was progressive disease (42%). At time of study closure, 14 patients (45%) remained on treatment; of these, 6 went on to receive ibrutinib in the commercial setting and 8 enrolled in a treatment extension study. Overall, 97% of patients experienced a treatment-emergent adverse event (TEAE), most commonly diarrhea (48%; grade ≥2, 13%) and pyrexia (35%; grade ≥2, 6%). Grade 3/4 TEAEs occurred in 81% of patients; the most common were neutropenia (16%), hypertension (10%), and anemia (10%). No deaths occurred due to AEs, and no patients died while on treatment. Ibrutinib dose was reduced in 5 patients (16%) due to an AE, and 2 patients (6%) discontinued treatment because of an AE. No patients experienced major hemorrhage or atrial fibrillation.
Conclusions: In this final analysis of the open-label substudy of the iNNOVATE trial, single-agent ibrutinib continued to show sustained efficacy in patients who had heavily pretreated, rituximab-refractory WM. Responses to ibrutinib were consistent across genotypes, although subgroup numbers were small. Ibrutinib maintained a manageable safety profile, and no new safety signals were identified with over 5 years of overall follow-up.
Trotman:Celgene: Research Funding; PCYC: Research Funding; Takeda: Research Funding; BeiGene: Research Funding; F. Hoffmann-La Roche: Research Funding. Buske:Roche, Janssen, Bayer, MSD: Research Funding; Roche, Janssen, AbbVie, Pfizer, Celltrion: Honoraria, Speakers Bureau; Morphosys: Membership on an entity's Board of Directors or advisory committees. Tedeschi:BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Department of Hematology Niguarda Hospital Milano: Current Employment; Sunesis: Consultancy; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen spa: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Matous:Celgene: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy. MacDonald:Roche Canada: Consultancy, Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria. Tam:BeiGene: Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Tournilhac:INNATE Pharma: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: Travel grant; Janssen: Consultancy, Honoraria, Other: Travel grant; GILEAD: Consultancy, Honoraria, Other: Travel Grant; ABBVIE: Consultancy, Honoraria, Other: Travle grant. Ma:Genentech: Consultancy, Honoraria; Novartis: Research Funding; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding; TG Therapeutics: Research Funding; Bioverativ: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Juno: Research Funding. Treon:Janssen: Consultancy, Other: Travel/accommodations/expenses; BeiGene: Consultancy; Bristol-Myers Squibb: Research Funding; BioGene: Other: Travel/accommodations/expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: Travel/accommodations/expenses, Research Funding. Oriol:Amgen: Consultancy, Speakers Bureau; Janssen: Consultancy; Sanofi: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ping:Pharmacyclics LLC, an AbbVie Company: Current Employment; AbbVie: Current equity holder in publicly-traded company. Briso:Pharmacyclics GmbH, an AbbVie Company: Current Employment; AbbVie: Current equity holder in publicly-traded company. Arango-Hisijara:Pharmacyclics LLC, an AbbVie Company: Current Employment; Bristol-Myers Squibb: Current equity holder in publicly-traded company; AbbVie: Current equity holder in publicly-traded company. Dimopoulos:Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal